40 explain how controlled substances are identified on their labels
› reg3aDepartment of Justice ADA Title III Regulation 28 CFR Part 36 ... Illegal use of drugs means the use of one or more drugs, the possession or distribution of which is unlawful under the Controlled Substances Act (21 U.S.C. 812). The term "illegal use of drugs'' does not include the use of a drug taken under supervision by a licensed health care professional, or other uses authorized by the Controlled ... Controlled Substance Flashcards | Quizlet Explain controlled substance schedule 1 - 5. Schedule 1 are highly addictive medications & schedule 5 are less addictive medications. Identify common administration routes of controlled substances. ... Explain handling of controlled substances. they are locked up in a double lock system. Identify when controlled substances are counted and by whom.
Hollywood Reporter #MeToo, Five Years Later: Why Time’s Up Imploded Hollywood’s most famous advocacy group is now a leaderless ghost organization, undone by conflicts of interest (and straight-up conflicts).

Explain how controlled substances are identified on their labels
Controlled Substance Veterinary Labels - LabelValue These veterinary labels are made with aggressive permanent adhesive and are great for prescription bottles, files, charts or records without loss of adhesion or print quality. Controlled Substance Labels: 500 Labels Per roll.375"x 1.625" 1" Core; Labels are 2-up; Aggressive permanent adhesive; Adhesive temperature range of -65 to 220 Fahrenheit CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 1302.02 Definitions. Any term contained in this part shall have the definition set forth in section 102 of the Act (21 U.S.C. 802) or part 1300 of this chapter. Sec. 1302.03 Symbol required; exceptions. (a) Each commercial container of a controlled substance (except for a controlled substance excepted by the Administrator pursuant to ... eCFR :: 29 CFR Part 1910 Subpart Z -- Toxic and Hazardous Substances (2) Acceptable ceiling concentrations. An employee's exposure to a substance listed in Table Z-2 shall not exceed at any time during an 8-hour shift the acceptable ceiling concentration limit given for the substance in the table, except for a time period, and up to a concentration not exceeding the maximum duration and concentration allowed in the column under “acceptable maximum …
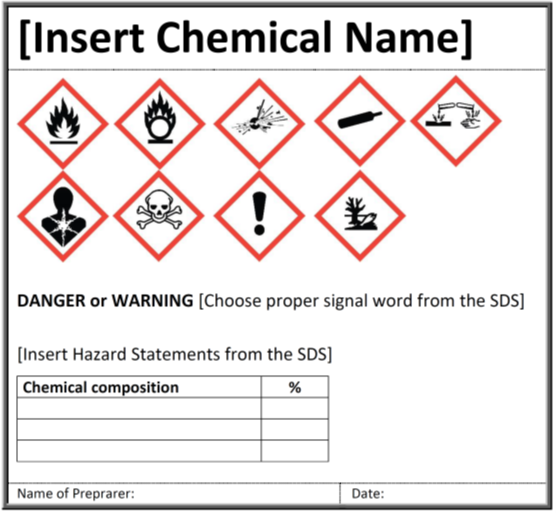
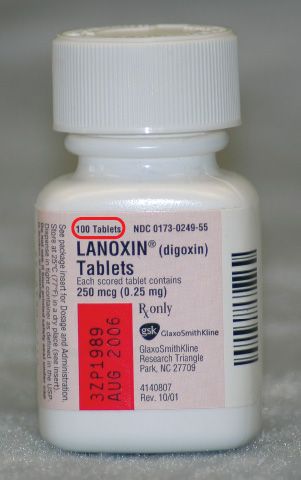
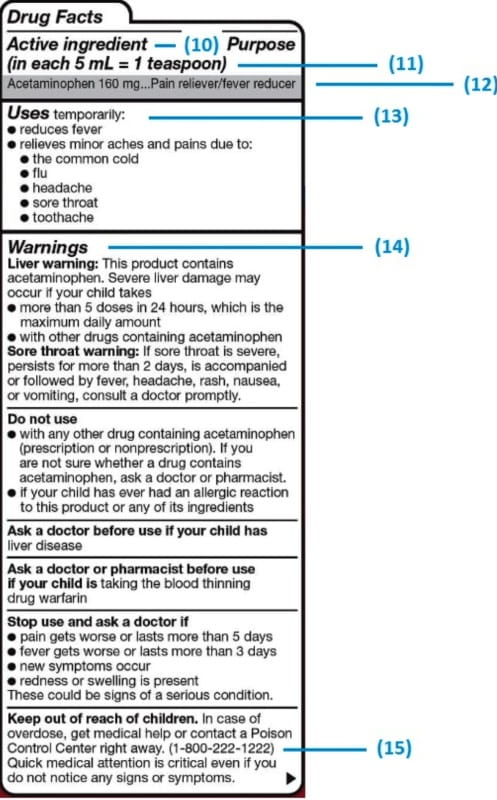
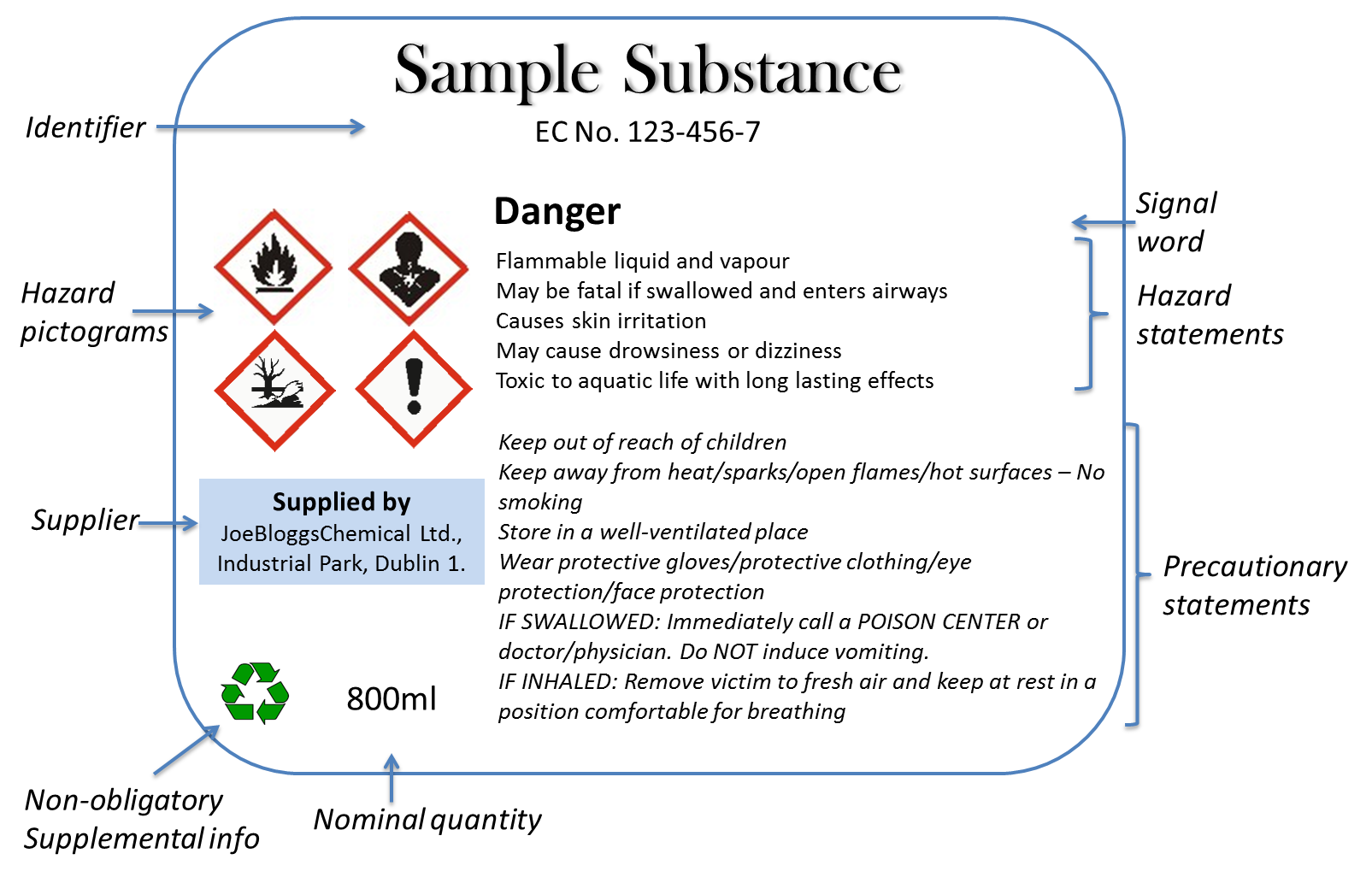
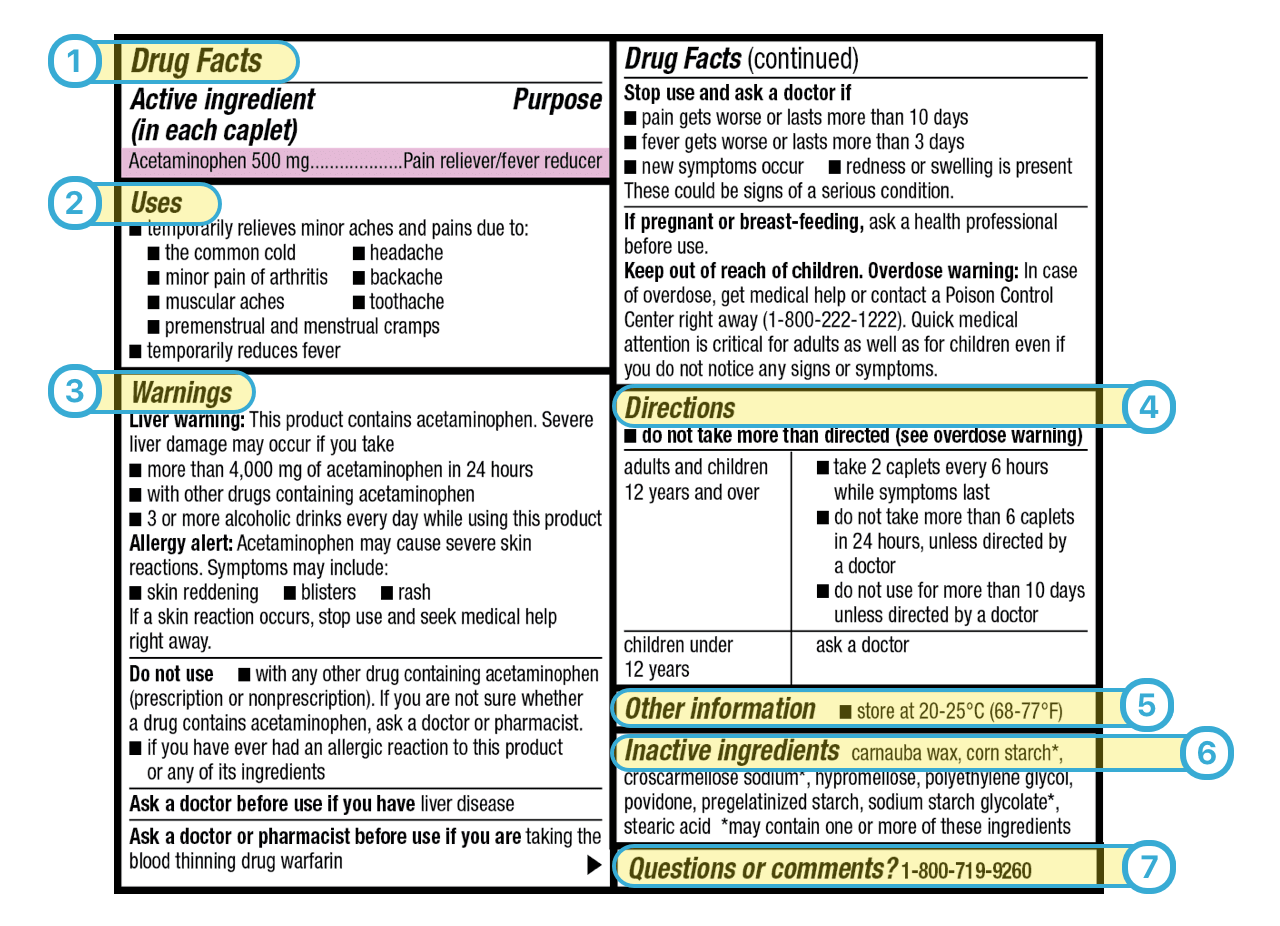
Explain how controlled substances are identified on their labels. What Information Should Be on Drug Labels? - MedicineNet The product and its use are described on the front and side panels of a drug container. There will be some indication of the product's classification next to the name. The Pr symbol indicates that the item is a prescription drug. No Pr denotes that no prescription is required to purchase it. Identification, Classification and Labelling of Chemicals ... All chemicals, both substances and preparations, should have a clear marking to indicate their identity. The packages and containers of dangerous substances and preparations should, in addition to marking only, to have a label with required information. eCFR :: 21 CFR Part 1302 -- Labeling and Packaging Requirements for ... ( a) Each commercial container of a controlled substance (except for a controlled substance excepted by the Administrator pursuant to § 1308.31 of this chapter) shall have printed on the label the symbol designating the schedule in which such controlled substance is listed. Controlled Substance Labels - Free Shipping | LabelValue This Controlled Substance Label measure 2" x 1" with 500 labels per roll in on a bright white paper material. Controlled substance labels for your patient and their specific medicine needs. A Controlled substance label is required by the Code of Federal Regulations 21 for schedule II, III, or IV controlled substances.
Federal Register :: Schedules of Controlled Substances; … Apr 12, 2022 · Pursuant to the Controlled Substances Act (CSA), under 21 U.S.C. 811(g)(3) ... Printed labels would need to indicate their status as a schedule III controlled substance. For example, the printed label would need to include “CIII” or “C-III.” ... If so, please explain with specific and quantified information as possible. 3. DEA estimates ... Controlled substances Flashcards | Quizlet Define controlled substances, CS are drugs and other substances that have been determined by federal and state to have potential for creating an addiction or dependency and for leading to various forms of abuse. Many CS are not recognized as drugs. Controlled drugs can be prescription or nonprescription, How do federal and state control CS, › current › title-29eCFR :: 29 CFR Part 1910 Subpart Z -- Toxic and Hazardous ... (2) Acceptable ceiling concentrations. An employee's exposure to a substance listed in Table Z-2 shall not exceed at any time during an 8-hour shift the acceptable ceiling concentration limit given for the substance in the table, except for a time period, and up to a concentration not exceeding the maximum duration and concentration allowed in the column under “acceptable maximum peak above ... ods.od.nih.gov › factsheets › Vitamind-HealthVitamin D - Health Professional Fact Sheet One systematic review and meta-analysis of 11 randomized, controlled trials published through 2018 of vitamin D supplementation alone (10–20 mcg [400–800 IU]/day or more at least every week or as rarely as once a year) for 9 months to 5 years found that the supplements provided no protection from fractures in 34,243 older adults .
HS140_Unit_8_Assignment.docx - HS140: Pharmacology Unit 8... - Course Hero HS140: Pharmacology Unit 8 Assignment Directions and Grading Rubric Applying Pharmacology Principles Unit Outcome(s) addressed in this Assignment: Explain drug agencies and their function; Describe and explain controlled substances; Explain proper medication administration principles; Explain poison control in older population patients. Course Outcome(s) assessed in this Assignment: HS140-4 ... The Hollywood Reporter #MeToo, Five Years Later: Why Time’s Up Imploded Hollywood’s most famous advocacy group is now a leaderless ghost organization, undone by conflicts of interest (and straight-up conflicts). Prescription of Controlled Substances: Benefits and Risks The Controlled Substance Act covers drug: Classification and regulation, according to their content and purpose. Manufacturing, Distribution, Exportation and sale, The Controlled Substance Act established five drug schedules and classified them to control their manufacture and distribution. nap.nationalacademies.org › read › 131654 Dimension 2: Crosscutting Concepts | A Framework for K-12 ... As their thinking advances, so too should their ability to recognize and apply more complex mathematical and statistical relationships in science. A sense of numerical quantity is an important part of the general “numeracy” (mathematics literacy) that is needed to interpret such relationships. Systems and System Models
4 Dimension 2: Crosscutting Concepts - The National Academies … Regardless of the labels or organizational schemes used in these documents, all of them stress that it is important for students to come to recognize the concepts common to so many areas of science and engineering. ... school. Thus, at the level of grades 3-5, matter flows and cycles can be tracked only in terms of the weight of the substances ...
Statutes & Constitution :View Statutes : Online Sunshine (15)(a) “Manufacture” means the production, preparation, propagation, compounding, cultivating, growing, conversion, or processing of a controlled substance, either directly or indirectly, by extraction from substances of natural origin, or independently by means of chemical synthesis, or by a combination of extraction and chemical synthesis, and includes any packaging of the …
EOF
WorkSafeBC 5.2 WHMIS: For hazardous substances covered by WHMIS, the worker must receive the education and training required by sections 5.6 and 5.7 of the Regulation. Section 5.6 deals with general (generic) requirements to ensure workers know among other things the elements of the WHMIS program, and the content required on labels and safety data sheets ...
› en › law-policyWorkSafeBC 5.2 WHMIS: For hazardous substances covered by WHMIS, the worker must receive the education and training required by sections 5.6 and 5.7 of the Regulation. Section 5.6 deals with general (generic) requirements to ensure workers know among other things the elements of the WHMIS program, and the content required on labels and safety data sheets ...
What Is a Controlled Substance? (DEA Drug Classifications) Controlled substances are drugs that are subject to strict government control because they may cause addiction or be misused. The government's control impacted how these substances are made, used, stored, and transported. Examples of controlled substances include: stimulants, opioids, hallucinogens, anabolic steroids, depressants,
The Controlled Substances Act - DEA The Controlled Substances Act (CSA) places all substances which were in some manner regulated under existing federal law into one of five schedules. This placement is based upon the substance's medical use, potential for abuse, and safety or dependence liability. More information can be found in Title 21 United States Code (USC) Controlled Substances Act.
The 5 Types Of Controlled Substances | Chemical Substance Regulation The schedules for controlled substances range from Schedule I to Schedule V. The lower the schedule, the greater the control is. This means that if someone is caught illicitly using, possessing, or selling a schedule 1 substance, the consequences will generally be greater than that of a schedule 5 substance.
Department of Justice ADA Title III Regulation 28 CFR Part 36 (1991) Illegal use of drugs means the use of one or more drugs, the possession or distribution of which is unlawful under the Controlled Substances Act (21 U.S.C. 812). The term "illegal use of drugs'' does not include the use of a drug taken under supervision by a licensed health care professional, or other uses authorized by the Controlled ...
How are controlled substances are identified on their labels ... How are controlled substances are identified on their labels? Wiki User. ∙ 2012-05-23 20:01:37. Add an answer. Want this question answered? Be notified when an answer is posted.
Vitamin D - Health Professional Fact Sheet - National Institutes of Health One systematic review and meta-analysis of 11 randomized, controlled trials published through 2018 of vitamin D supplementation alone (10–20 mcg [400–800 IU]/day or more at least every week or as rarely as once a year) for 9 months to 5 years found that the supplements provided no protection from fractures in 34,243 older adults .
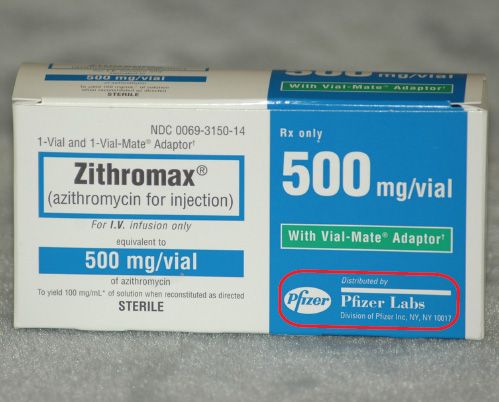
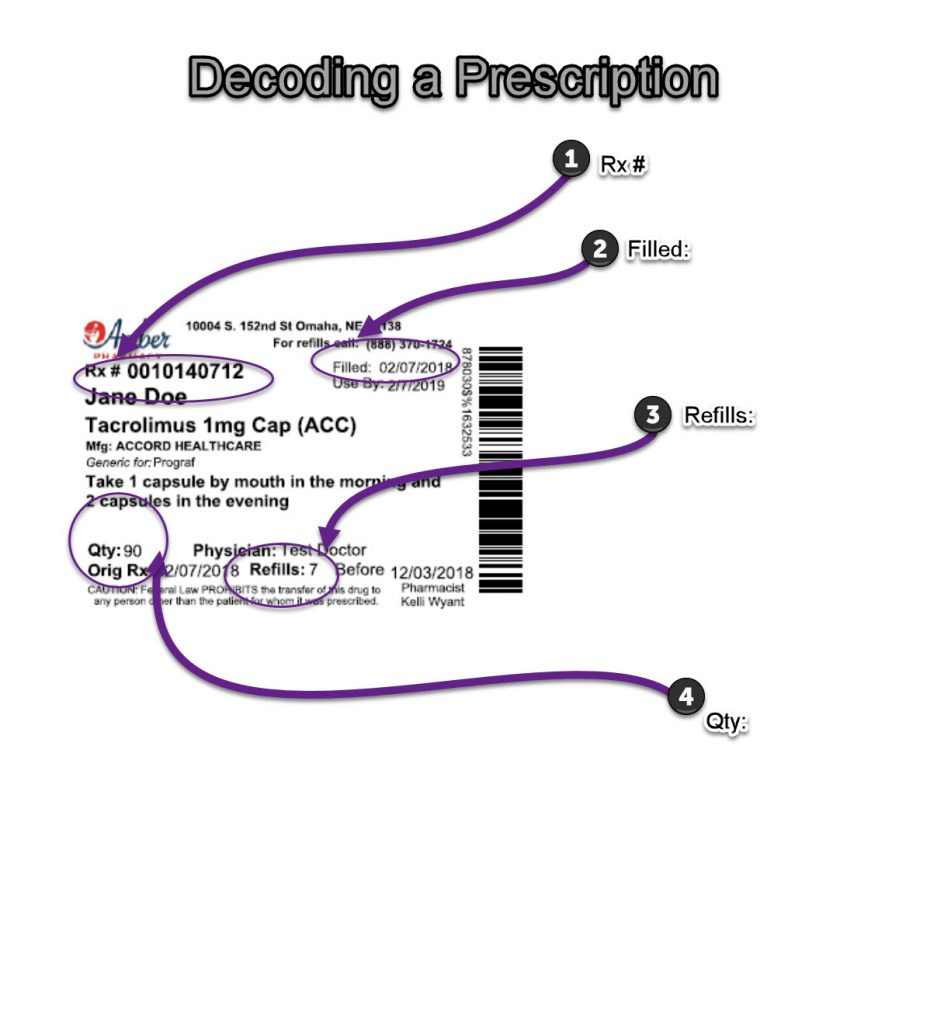
Solved How are controlled substances identified by | Chegg.com How are controlled substances identified by pharmaceutical companies (on the dispensing bottle (the large quantities purchased by pharmacies or veterinarians), not the; Question: How are controlled substances identified by pharmaceutical companies (on the dispensing bottle (the large quantities purchased by pharmacies or veterinarians), not the
453 Controlled Substances and Drugs | Postal Explorer - USPS Controlled substances. The inner packaging of any mailpiece containing a mailable controlled substance must be marked and sealed in accordance with the applicable provisions and regulations of the Controlled Substances Act (see 453.11). The inner packaging is also labeled to show the prescription number and the name and address of the pharmacy ...
What Is a Controlled Substance? - Verywell Mind Controlled substances are classified into schedules based on their medical value and potential for abuse. Schedule 1 drugs have no federally recognized medical purpose and high risk for dependence and abuse. Schedule 2 drugs may have some purpose in restricted medical settings.
Medication - Wikipedia A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.Drugs are classified in multiple …
Controlled substances"means any drug or substance identified Sample ... Controlled substances"means any drug or substance identified. in Schedules I through IV of the Controlled Substances Act or its implementing regulations and includes, but is not limited to, marijuana,...
quiz 7.docx - What are Controlled Substances and explain... What are Controlled Substances and explain nursing responsibilities as it relates to controlled substance.?--/8 Points Controlled substances are illegal or prescription drugs regulated by the Controlled Substances Act (CSA) in the United States. Recognizing the potential that certain medications have for abuse and dependence, Congress enacted the CSA as part of the Comprehensive Drug Abuse ...
› documents › 2022/04/12Federal Register :: Schedules of Controlled Substances ... Apr 12, 2022 · Any person who becomes registered with DEA must take an initial inventory of all stocks of controlled substances (including butalbital products) on hand on the date the registrant first engages in the handling of controlled substances, pursuant to 21 U.S.C. 827 and 958 and in accordance with 21 CFR 1304.03, 1304.04, and 1304.11.
Controlled Substances | Veterian Key Controlled substances include opiates (narcotics), barbiturates, hallucinogens (e.g., ketamine), amphetamines, and other addictive and habituating drugs. Class I drugs have the highest abuse potential; therefore medical use of these substances is not allowed in the United States.
eCFR :: 29 CFR Part 1910 Subpart Z -- Toxic and Hazardous Substances (2) Acceptable ceiling concentrations. An employee's exposure to a substance listed in Table Z-2 shall not exceed at any time during an 8-hour shift the acceptable ceiling concentration limit given for the substance in the table, except for a time period, and up to a concentration not exceeding the maximum duration and concentration allowed in the column under “acceptable maximum …
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 1302.02 Definitions. Any term contained in this part shall have the definition set forth in section 102 of the Act (21 U.S.C. 802) or part 1300 of this chapter. Sec. 1302.03 Symbol required; exceptions. (a) Each commercial container of a controlled substance (except for a controlled substance excepted by the Administrator pursuant to ...
Controlled Substance Veterinary Labels - LabelValue These veterinary labels are made with aggressive permanent adhesive and are great for prescription bottles, files, charts or records without loss of adhesion or print quality. Controlled Substance Labels: 500 Labels Per roll.375"x 1.625" 1" Core; Labels are 2-up; Aggressive permanent adhesive; Adhesive temperature range of -65 to 220 Fahrenheit




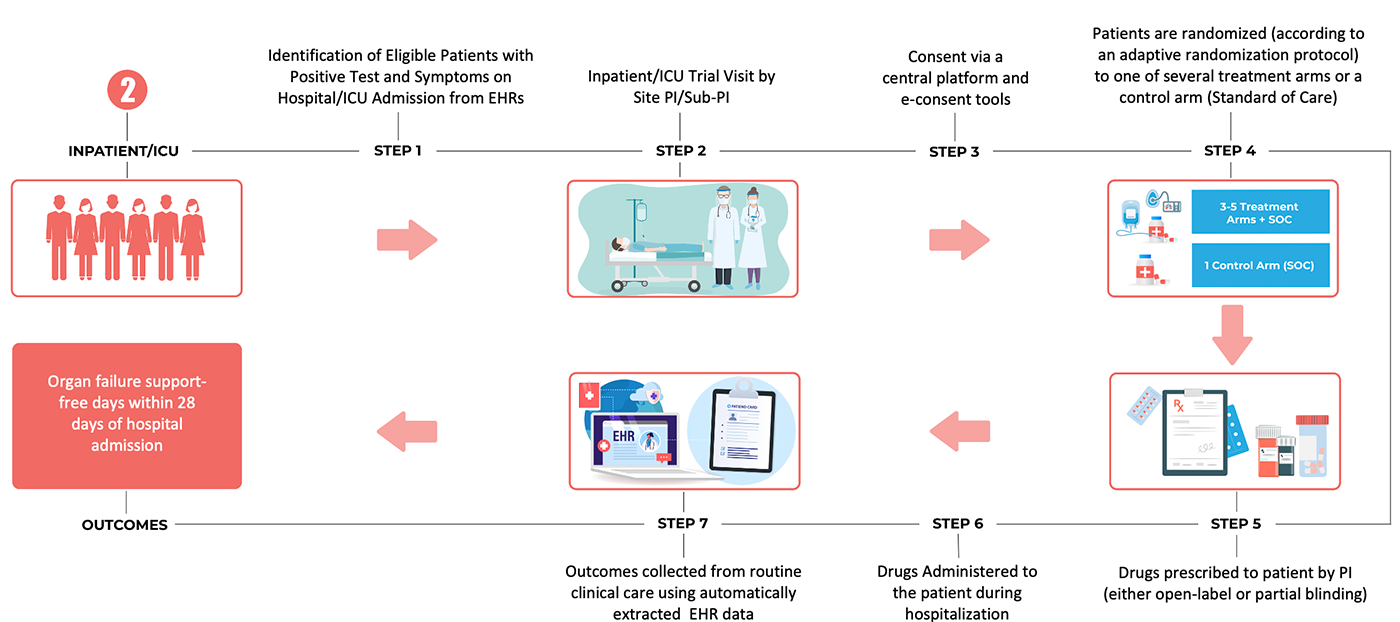
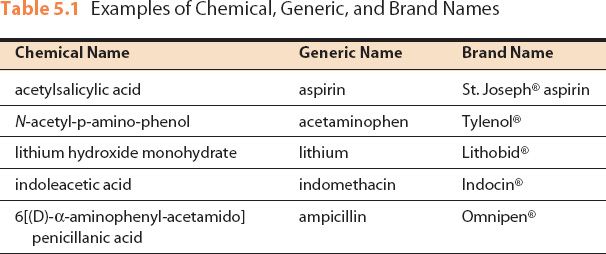




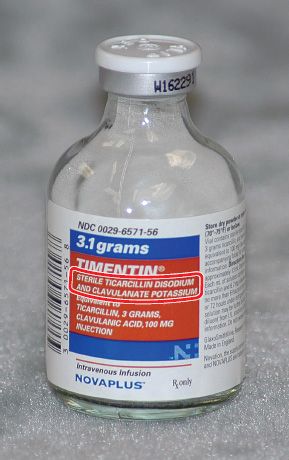








Post a Comment for "40 explain how controlled substances are identified on their labels"